
Chlorine Dioxide Bleaching
Chlorine dioxide is an excellent bleaching chemical because it is very selective for lignin. Because of this, its use has expanded greatly over the last few decades. This course discusses the purpose of chlorine dioxide bleaching and the basics of chlorine dioxide bleaching chemistry. It also describes the equipment used in chlorine dioxide bleaching, as well as the typical ECF bleaching sequences such D0, D1, and D2 along with their differences.
Request a demoCourse Details
Learning Objectives
• Describe the purpose of chlorine dioxide bleaching
• Describe the basics of chlorine dioxide bleaching chemistry
• Explain how pH affects chlorine dioxide bleaching
• Explain how temperature affects chlorine dioxide bleaching
• Identify and describe equipment used for chlorine dioxide bleaching
• Identify typical ECF bleaching sequences
• Identify and describe differences between D0, D1, and D2 stages
• Describe modification required for hardwood bleaching
• Describe the safety hazards and safety guidelines for chlorine dioxide
Specs
| Course Level | Intermediate |
| Languages | English, Portuguese, French, Polish, Russian |
| Compatibility | Audio, Video |
| Based on: | Industry Standards and Best Practices |
Key Questions
Why is chlorine dioxide generated onsite?
Chlorine dioxide is unstable as a gas and decomposes explosively at low pressures
What is ECF bleaching?
ECF stands for elemental chlorine (Cl2) free. Bleaching with chlorine dioxide is considered ECF
What is the best pH for a D0 stage?
The best results for a D0 stage are achieved with a final pH 3-4
How should chlorine dioxide be distributed among the different D stages.
The chlorine dioxide should be distributed to optimize the effect on brightness. A common distribution is 40-50% in D0, 30-40% in D1 and 15-25% in D2. If there are only two stages, ClO2 addition is usually split evenly.
What are some safety concerns with using chlorine dioxide?
One of the biggest concerns is the potential for explosions. Also, chlorine dioxide can be a serious threat to the respiratory system and exposure to high concentrations can be fatal.
Sample Video Transcript
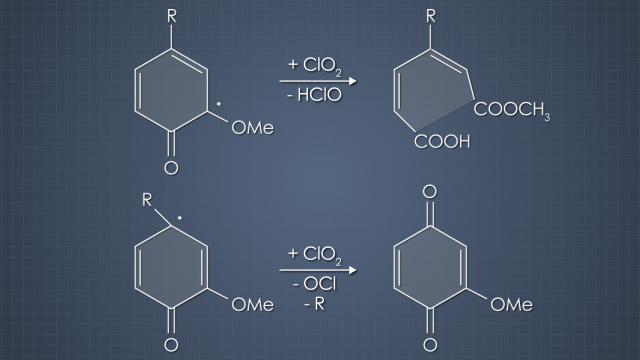
Chlorine dioxide is an excellent bleaching chemical because it is very selective for lignin. However, it is relatively expensive, so chlorine dioxide was originally used in later bleaching stages where there is low lignin content to produce pulp with a high, stable brightness without compromising strength. Over the last few decades, the use of chlorine dioxide has expanded. At first, chlorine dioxide was used to protect pulp strength by replacing 5% to 10% of the chlorine. It was later found that substituting up to 50% chlorine dioxide improved the delignification efficiency. To reduce the environmental impact of using elemental chlorine, chlorine dioxide has essentially replaced chlorine and become the foundation of elemental chlorine-free, or ECF bleaching. Because chlorine dioxide reacts much differently with pulp than chlorine, the production of harmful dioxins is less than 2% of that created by chlorine.
Course Applies To
Demos + Pricing
Learn more about our courses, get pricing, and see our platform.